GOST 23116.1-78
THE HIGH-PURITY CADMIUMMethod of spectrographic determination of aluminum, bismuth, Cadmium high purity. Method of spectrographic of aluminium, bismuth, This standard specifies the method of spectrographic determination of impurities in cadmium of high purity in the following range mass fraction, %: |
| aluminium | from | 1·10-6 | to | 6·10-5 |
| bismuth | « | 1·10-6 | « | 5·10-5 |
| iron | « | 2·10-6 | « | 1 10-3 |
| indium | « | 1·10-6 | « | 5·10-5 |
| cobalt | « | 1·10-6 | « | 5·10-5 |
| copper | « | 7·10-7 | « | 5·10-4 |
| manganese | « | 7·10-7 | « | 1 10-4 |
| arsenic | « | 2·10-5 | « | 1 10-3 |
| Nickel | « | 1·10-6 | « | 5·10-4 |
| tin | « | 1·10-6 | « | 5·10-4 |
| lead | « | 7·10-6 | « | 1 10-3 |
| antimony | « | 7·10-6 | « | 5·10-4 |
| silver | « | 3·10-7 | « | 3·10-5 |
The method is based on the preconcetration of impurities by vacuum distillation of the bulk of cadmium and spectrographic analysis of the resulting concentrate.
To increase the efficiency of extraction of impurities in the concentrate are added cadmium alloy cadmium-tellurium.
(Changed edition, Rev. No. 1).
1. GENERAL REQUIREMENTS
1.1. General requirements for method of analysis and safety requirements according to GOST 23116.0.
(Changed edition, Rev. No. 1).
2. APPARATUS, REAGENTS AND SOLUTIONS
The quartz spectrograph medium dispersion of any type, allowing a single exposure to range from 230,0 up to 400,0 nm, being equipped with an illumination system of the slit.
The generator is activated the arc AC.
Spectromancer of PS-18.
Microphotometer designed to measure pucherani spectral lines (complete set).
Grinding machine carbon electrodes.
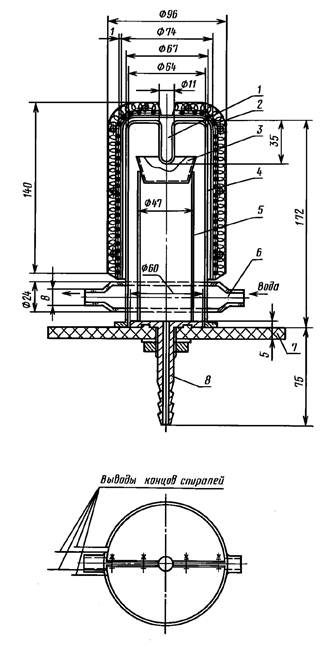
1 — input thermometer;2 — heater 500 watts; 3 — Cup sample; 4 — quartz
vacuum chamber; 5- stand; 6- caisson; 7 — strip of vacuum rubber; 8 — vacuum-fitting
Libra torsion bar type W with a weighing error of no more than 0.001 g.
Analytical scale with a weighing error of no more than 0,0002 g.
Muffle furnace with temperature up to 750 °C.
The quartz device for the distillation of reagents.
Boxes of organic glass.
Electric tile according to GOST 14919, covered with a quartz plate.
The infrared lamp with a laboratory autotransformer.
Installation for vacuum distillation of the base (see drawing) or other similar structure.
Vacuum gauge type W-2 to measure the vacuum of 6.67 to 26.66 PA (0.05 to 0.2 mm Hg. calendar).
Forevacuum pump type EXT-46/M or 3-HBP-1D.
Hoses vacuum.
A laboratory autotransformer RNO-250−2.
Thermocouple or thermometer 500 °C.
Valves, vacuum, double and triple.
Quartz ware (cups, glasses, etc.).
Flasks with graduated neck according to GOST 12738.
Pipettes according to GOST 29227 with a capacity of 1, 2, 5 and 10 cm3.
Electrodes, carbon, OS. h, 6 mm in diameter, with the crater size was 4x3 mm and contradictory length 30 — 50 mm, one end sharpened to a truncated cone with ground diameter of 1.5 mm.
Distilled water according to GOST 6709, double-distilled in quartz apparatus or purified on a column of ionization.
Graphite powder of high purity according to GOST 23463 or obtained from carbon electrodes of high purity.
Nitric acid of high purity according to GOST 11125.
Rectified ethyl alcohol according to GOST 18300.
Tellurium brand according to normative-technical documentation.
Cadmium brand Кд0000 according to GOST 22860.
Alloy cadmium-tellurium. The content of tellurium in the alloy 0,04 — 0,07%; the alloy is prepared as follows: in a quartz ampoule put the sample of cadmium and tellurium. Pumped from the ampoule in the air to 1.33 PA (1·10-2 mm Hg. calendar), and the vial was sealed and heated in a muffle furnace at 700 °C for 6 — 7 h, every 30 min stirring thoroughly. Then the ampoule is opened and through the hole, the melt poured a thin stream into the glass of water. The granules thus obtained alloy is washed in ammonia water, then in water and dried. Sodium chloride of high purity. Tartaric acid according to GOST 5817. The aqueous ammonia of high purity according to GOST 24147.
Ammonia of high purity 17−4 mark.
Diethyl ether (medical).
Grease vacuum.
Oxalic acid according to GOST 22180.
Sulfuric acid according to GOST 4204.
Tin according to GOST 860.
Antimony according to GOST 1089.
A buffer mixture consisting of powder of graphite with the addition of 0.5% of sodium chloride.
Solutions of the identified elements are given in table. 1.
Samples comparison. The basic sample containing 1·10-3 % copper and silver, 3·10-2 % of lead, 3 10-1 % arsenic, and other elements to 1·10-2 %; prepared as follows: in a quartz Cup with a capacity of 150 cm3 pour 10 g of powder of graphite and is administered by 1 cm3 of solutions of copper, silver, aluminum, Nickel, indium, bismuth, iron, cobalt, manganese, tin, antimony, 3 cm3 of a solution of lead and 30 cm3 of arsenic. The mixture is stirred quartz wand, dried under a heat lamp and then calcined on a tile to remove odor of nitric acid. Prepared core sample is thoroughly ground in a mortar made of organic glass. The first comparison sample is prepared by dilution of main sample diluent 10 times. Then by serial dilution of each newly prepared sample diluent three times is preparing a series of working samples. Prepared samples for plotting and diluent stored in buksh or jars with screw caps.
The diluent is prepared as follows: in a quartz Cup with a capacity of 100 cm3 were placed 50 g of powdered graphite and nakatyvaet 5 cm3 of solutions of tellurium and cadmium. The mixture is dried on the tile to remove the smell of nitric acid and then injected her with 5 cm3 of sodium chloride solution. Re-dried, mixed and mulled.
Spectrographic plates of type II.
Preparation of standard solutions see table. 1.
Table 1
| Item | The contents of the element, mg/cm3 | The method of solution preparation |
| Copper | 0,1 | The calculated weighed portion of metal, oxide, carbonate or nitrate salt dissolved in nitric acid |
| Silver | 0,1 | |
| Aluminium | 1,0 | |
| Nickel | 1,0 | |
| Indium | 1,0 | |
| Bismuth | 1,0 | |
| Lead | 1,0 | |
| Iron | 1,0 | |
| Cobalt | 1,0 | |
| Tellurium | 20,0 | |
| Cadmium | 50,0 | |
| Manganese | 1,0 | Dissolved in water, anhydrous manganese sulfate |
| Arsenic | 1,0 | Is dissolved in water arsenous acid anhydride with a few drops of ammonia in low heat |
| Tin | 1,0 | 100 mg of finely divided tin is placed in a beaker with a capacity of 50 cm3 and add 2 cm3 of nitric acid. After the transition of the whole sample in metalogeny acid to the beaker was added 20 cm3 of water and 4 g of oxalic acid. The solution was transferred to a volumetric flask with a capacity of 100 cm3. The glass is washed several times with water for complete dissolution of oxalic acid. The washings decanted into the same volumetric flask |
| Antimony | 1,0 | 100 mg of finely divided antimony is placed in a beaker with a capacity of 50 cm3 and add 2 cm3 of nitric acid. The glass is heated to the transfer of all of the antimony in measuremenu acid. Not stopping heating, add 10 cm3 of tartaric acid. The obtained clear solution is transferred to a volumetric flask with a capacity of 100 cm3 |
| Sodium chloride | 50,0 | The calculated charge is dissolved in water |
Sec. 2. (Changed edition, Rev. No. 1, 2).
3. ANALYSIS
3.1. To obtain a concentrate of impurities
3.1.1. Before starting, you must carefully prepare the installation for vacuum distillation of cadmium. To do this, using cotton wool soaked in diethyl ether, remove vacuum grease from the ground, dissolved in nitric acid residues of cadmium and immersed for 10 min the entire installation in a nitrating mixture consisting of three parts sulfuric and one part of nitric acid, the temperature of which should be close to the boiling point. Then thrice washed with water and dried in a drying Cabinet at a temperature of approximately 170 °C.
3.1.2. Cadmium analysis comes in the form of pigs weighing 20 — 40 g, diameter 15 — 25 mm, height of 10 — 20 mm. Before operation it is kept in solution ammonia to dissolve the oxide film, washed twice with water and dried. The thus prepared sample is placed in a quartz Cup with a capacity of 50 cm3 by adding 500 mg of alloy cadmium-tellurium. The Cup with the sample mounted on a quartz stand in a vacuum chamber, put a quartz dome, a cone which is smeared with vacuum grease. Hood grind to a vacuum rubber. Create a vacuum of 6.67 — 13,33 PA (0,05 — 0,1 mm Hg. calendar). To the fridge to attach the hoses for the inflow and discharge of water and include heating. Cooling should be insignificant.
Vacuum distillation of cadmium is carried out at 380 — 410 °C for 2 — 4 h. After removal of the foundations of the heater is removed, block the vacuum, open the valve for air supply, remove the vacuum chamber and into a Cup add 100 mg of the buffer. The mixture was stirred for 5 — 7 min. the enrichment Coefficient (K) is calculated according to the formula
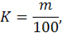
where t is the sample mass, mg;
100 — mass of the concentrate, mg.
To carry out control experience in a quartz Cup with a capacity of 50 cm3 is placed 500 mg of the alloy, cadmium-tellurium and carry out the Stripping of cadmium in a vacuum under the same conditions as for the sample, for 20 — 25 min. To the residue was added 100 mg of buffer and grind thoroughly.
(Changed edition, Rev. No. 1).
3.2. Spectral analysis of the concentrate
Prepared from concentrates and Supervisory experience, as well as from the samples of the comparison on a torsion scale for three selected sample of 20 mg and placed in craters of the carbon electrodes. Carbon electrodes are pre-fired in an arc to an alternating current power 15 A or DC power 10 A for 10 s.
The spectra are photographed on a quartz spectrograph with slit width of 0.015 mm on the spectrographic photoplates of the type II. Evaporation of the sample and the excitation spectrum is carried out in the arc of an alternating current power 14 A. the exposure Time of 15 s. To construct the characteristic curve of photographic plates simultaneously with the samples and the samples of the comparison spectrum photographed through a nine-iron platinum reliever.
(Changed edition, Rev. No. 2).
4. PROCESSING OF THE RESULTS
4.1. In the spectrogram, using microphotometry measure the blackening of analytical lines of the determined elements and the background to the right or to the left of them. Build a response curve
photographic plates and find the value of lg (Il+f) and lgIf. Calculate the value 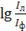 , find the average value of this quantity for the three spectra of each sample to graph, concentrate and control experience. The calibration graphs are built in coordinates
, find the average value of this quantity for the three spectra of each sample to graph, concentrate and control experience. The calibration graphs are built in coordinates 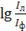 , lgC, where C — concentration of the analyzed element in the sample comparisons. For the calibration chart find the content of impurities in the concentrate and the control experiment. Fraction of total mass of element (X) in percent is calculated by the formula
, lgC, where C — concentration of the analyzed element in the sample comparisons. For the calibration chart find the content of impurities in the concentrate and the control experiment. Fraction of total mass of element (X) in percent is calculated by the formula
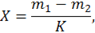
where t1 — mass fraction of the element in the concentrate, %;
t2 — mass fraction of the element in a control experiment, %;
K — coefficient of enrichment.
The final result of the analysis taking the arithmetic mean of two parallel definitions (each definition derived from three spectrograms).
The difference between the two results of parallel measurements with a confidence probability P = 0.95 does not exceed the value of permissible differences (dn) calculated by the formulas:
dn = 0.3 mm 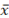 for bismuth, manganese, copper, tin, antimony, and silver;
for bismuth, manganese, copper, tin, antimony, and silver;
dn = 0,4 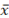 — for cobalt, arsenic, Nickel and lead;
— for cobalt, arsenic, Nickel and lead;
dn = 0,5 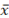 — for aluminium, iron and India,
— for aluminium, iron and India,
where 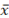 is the arithmetic mean of the two comparable results of parallel measurements.
is the arithmetic mean of the two comparable results of parallel measurements.
The difference between two results of analysis of the same samples with confidence probability P = 0.95 does not exceed the value of permissible divergence (da), calculated by the formula:
da = 0,3 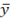 - for bismuth, manganese, copper, tin, antimony, and silver;
- for bismuth, manganese, copper, tin, antimony, and silver;
da = 0,4 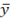 - for cobalt, arsenic, Nickel and lead;
- for cobalt, arsenic, Nickel and lead;
da = 0,5 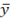 - for aluminium, iron and India,
- for aluminium, iron and India,
where 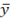 is the arithmetic mean of the two comparable test results.
is the arithmetic mean of the two comparable test results.
Photometric the following analytical lines (wavelengths in nm):
aluminium — Al I 308,22;
bismuth — Bi I 306,79;
iron — Fe I or Fe I 302,06 271,9;
indium — 325,61 In I or In I 303,94;
cobalt — I 341,23 or I 304,40;
copper — si I or si 327,40 I 324,75;
manganese — Mn I 279,83;
arsenic — As I 234,98;
Nickel — Ni I Ni I 305,08 or 341,48
tin — Sn I or Sn I 303,41 284,0;
lead — Pb I 283,31;
antimony — Sb I 259,80;
silver — Ag I 328,07.
(Changed edition, Rev. No. 1, 2).
4.2. Allowable absolute differences between the results of two parallel measurements calculated for a confidence probability P = 0,95, shall not exceed the values specified in table. 2.
Table 2
| The designated element | The mass fraction of the element, % | The coefficient of variation, % | The absolute allowable difference, % |
| Aluminium | 2·10-6 to 6·10-6 SV. 6·10-6 «2·10-5 «2·10-5» 6·10-5 |
20,0 | 1·10-6 3·10-6 1·10-5 |
| Bismuth | 2·10-6 «5·10-6 SV. 5·10-6 «1·10-5 «1·10-5» 5·10-5 |
10,0 | 6·10-7 2·10-6 3·10-6 |
| Iron | From 5·10-6 «2·10-5 SV. 2·10-5 «1·10-4 «1·10-4» 5·10-4 «5·10-4» 1 to 10−3 |
20,0 | 3·10-6 1·10-5 6·10-5 3·10-4 |
| Cobalt | 2·10-6 to 5·10-6 SV. 5·10-6 «1·10-5 «1·10-5» 5·10-5 |
15,0 | 8·10-7 2·10-6 4·10-6 |
| Copper | From 1·10-6 «5·10-6 SV. 5·10-6 «1·10-5 «1·10-5» 5·10-5 «5·10-5» 3·10-4 «3·10-4» 5·10-4 |
10,0 | 3·10-7 2·10-6 3·10-6 2·10-5 8·10-5 |
| Indium | 2·10-6 «5·10-6 SV. 5·10-6 «1·10-5 «1·10-5» 5·10-5 |
20,0 | 1·10-6 3·10-6 6·10-6 |
| Manganese | From 5·10-6 «1·10-5 SV. 1·10-5 «3·10-5 «3·10-5 » 1·10-4 |
10,0 | 2·10-6 3·10-6 8·10-6 |
| Nickel | 2·10-6 «6·10-6 SV. 6·10-6 «2·10-5 «2·10-5» 1·10-4 «1·10-4» 5·10-4 |
15,0 | 8·10-7 3·10-6 8·10-6 4·10-5 |
| Tin | 2·10-6 «5·10-6 SV. 5·10-6 «1·10-5 «1·10-5» 5·10-5 «5·10-5» 1·10-4 «1·10-4» 5·10-4 |
10,0 | 6·10-7 2·10-6 3·10-6 2·10-5 3·10-5 |
| Lead | From 1·10-5 «3·10-5 SV. 3·10-5 «1·10-4 «1·10-4» 5·10-4 «5·10-4» 1·10-3 |
15,0 | 4·10-6 1·10-5 4·10-5 2·10-4 |
| Antimony | From 1·10-5 «5·10-5 SV. 5·10-5 «1·10-4 «1·10-4» 5·10-4 |
10,0 | 3·10-6 2·10-5 3·10-5 |
| Silver | From 5·10-7 «1·10-6 SV. 1·10-6 «5·10-6 «5·10-6» 1·10-5 «1·10-5» 5·10-5 «5·10-5» 1·10-4 |
10,0 | 2·10-7 3·10-7 2·10-6 3·10-6 2·10-5 |
| Arsenic | 4·10-5 «1·10-4 SV. 1·10-4 «5·10-4 «5·10-4» 1·10-3 |
15,0 | 2·10-5 4·10-5 2·10-4 |
INFORMATION DATA
1. DEVELOPED AND INTRODUCED by the Ministry of nonferrous metallurgy of the USSR
2. APPROVED AND promulgated by the Decree of the State Committee of standards of Ministerial Council of the USSR from
3. REFERENCE NORMATIVE AND TECHNICAL DOCUMENTS
| The designation of the reference document referenced | Section number, paragraph |
| GOST 860−75 | 2 |
| GOST 1089−82 | 2 |
| GOST 4204−77 | 2 |
| GOST 5817−77 | 2 |
| GOST 6709−72 | 2 |
| GOST 11125−84 | 2 |
| GOST 12738−77 | 2 |
| GOST 14919−83 | 2 |
| GOST 18300−87 | 2 |
| GOST 22180−76 | 2 |
| GOST 22860−93 | 2 |
| GOST 23116.0−83 | 1.1 |
| GOST 23463−79 | 2 |
| GOST 24147−80 | 2 |
| GOST 29227−91 | 2 |
4. Limitation of actions taken by Protocol No. 3−93 Interstate Council for standardization, Metrology and certification (ICS 5−6-93)
5. EDITION with Amendments No. 1, 2 approved in December 1983, December 1988 (IUS 4−84, 3−89)
GOST







